
Members of the same group have the same number of valence electrons and have similar properties as those valence electrons behave similarly.
ORBITAL DIAGRAMS FOR ALL ELEMENTS FULL
As a result, the nuclear charge actually felt by outer electrons is diminished, and we say that the outer electrons are shielded from the full charge of the nucleus by the inner electrons.Ģ3 Periodic Properties & Valence Electrons Outer electrons are attracted toward the nucleus by the nuclear charge but are pushed away by the repulsion of inner electrons. Think of picking up iron filings or paper clips with a magnet, eventually the magnet can’t attract any more filings as it is “shielded”.Ģ2 Figure: 05-16 Title: Effective nuclear charge (Z) Caption: Figure 5.16 The origin of electron shielding and Zeff. So the shielded valence electrons don't feel the full nuclear charge, Z, they instead feel what we call Zeff, or the effective nuclear charge.

So they are removed more easily than core electrons (inner shell electrons).Ģ1 Periodic Properties & Valence ElectronsĪlso, the core electrons with their negative charges block or insulate or "shield" the valence electrons from feeling the full nuclear charge. Thus they are not as tightly held to the nucleus.

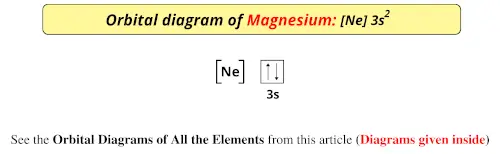
Valence electrons are outer shell electrons and so are furthest from the nucleus (generally). And valence electrons are responsible for chemical properties.Ģ0 Periodic Properties & Valence Electrons Why? They have the same number of valence electrons, or their outermost electron configurations are the same. Members of a group have similar chemical properties. ns 2s -> 2p -> 3s -> 3p -> 4s -> 3d -> 4p, and so on.ġ8 Electron Configurations and Periodic Propertiesġ9 Periodic Properties & Valence Electrons The letter is the orbital type or the sublevel type The number of lines beside the sublevel is how many orbitals there are on that sublevel Do you notice any pattern?ĥ Orbital Diagrams For every n value, the s orbital is the lowest energy. Hund’s Rule Aufbau Principle Pauli’s Exclusion Principle There is also an order which we follow in placing electrons in an orbital energy diagram.Ĥ Orbital Diagrams The number is the energy level (n value) There are rules for placing electrons in orbitals. Every orbital may hold up to 2 electrons These 2 electrons have opposite spins: an up spin and a down spin You can determine how many electrons are possible for an energy level by 2n2 If n=1, only 2 electrons are possible If n=2, 8 electrons are possible If n=3, 18 electrons are possible


 0 kommentar(er)
0 kommentar(er)
