
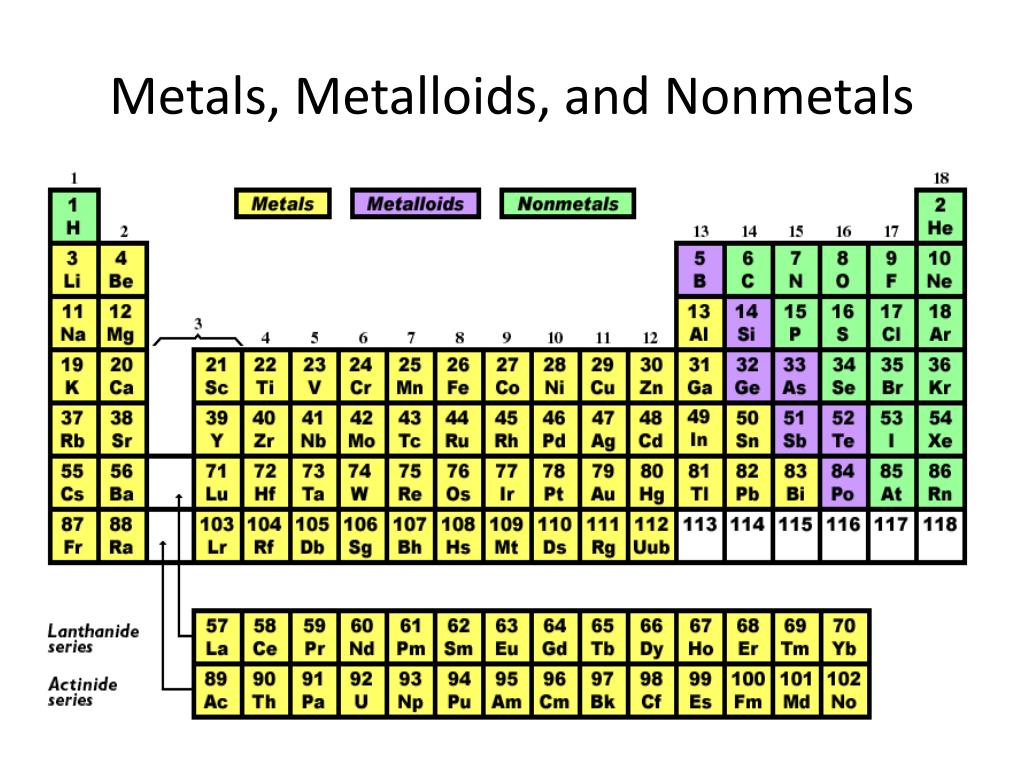
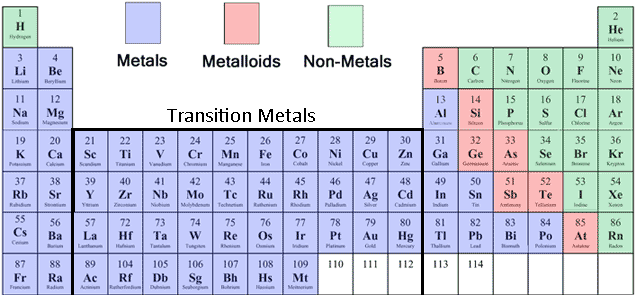
Because of their properties, metals are grouped according to their positions in the periodic table. Additionally, these factors are prone to get rid of electrons, and might kind ions with a positive charge or no cost whatsoever. Precious metals demonstrate a variety of attributes, including electric powered conductivity, lustre, and malleability. This class consists of both alloys and nonmetals. The principle band of the periodic dinner table comprises the weather with s and p electron designs. The attributes of move materials are very important for his or her use within environmental surroundings, along with their distinctive properties also assist to clarify their recognition amid experts. Their least expensive boiling and melting details are associated with low-alloys. The best melting and boiling details are associated with changeover materials. These reactive elements often type ores with many other metals and non-precious metals. From the Routine Kitchen table, alkali metals are classified as the s-obstruct aspects.įrom the Routine Kitchen table, transition metals are in a natural way plentiful aspects within the earth's crust. They also become more reactive in water, as their reactivity increases. They are usually present in salts, where you can system-focused cubic construction. These metals are most reactive when in contact withair and water. Your browser does not support the audio element.The brand alkali alloys comes from the Arabic phrase al-qali, that means ashes.
#Metals on the periodic table free
They are not found in nature as a free element, but generally as salts.When exposed to air, they tarnish due to oxidation.They are soft enough to be cut with a knife.
#Metals on the periodic table full
They all have one valence electron in the outermost shell which they seek to lose in order to have a full outer shell.What are the similar properties of alkali metals?Īlkali metals share many similar properties including: Click the links or see below for more details on each. The elements of the alkali metals include lithium, sodium, potassium, rubidium, cesium, and francium.

Hydrogen and the alkali metals make up the group 1 elements of the periodic table. The only element in the first column that is not usually considered an alkali metal is hydrogen. They are all in the first column of the periodic table. The alkali metals are a group of elements in the periodic table.


 0 kommentar(er)
0 kommentar(er)
